40 dot diagram for calcium
A step-by-step explanation of how to draw the Lewis dot structure for Ca (Calcium). I show you where Calcium is on the periodic table and how to determine h... Draw Ca in the center. Draw 2 lines from it going in opposite directions. Put a Cl at the end.Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in These diagrams are used as a shorthand notation to show the number of valence electrons in an atom.
Question: Draw a dot- and- cross diagram for calcium atom, showing the electrons in the outermost shells. Calcium is in Group II --> 2 valence electrons. Dot- and- cross diagram of calcium atom: Part 2: Dot- and- cross diagram for Ionic Compounds Recap - Ionic compounds are made of metals and non- metals.
Dot diagram for calcium
The Lewis structure shows the calcium with no dots (electrons), and the chlorine ions with a complete octet. Notice the placement of the charge notation on the ions. 3. The Ca and Cls are near each other, but the two dots (electrons) from each Cl should not be interpreted as a covalent bond. The final Lewis dot structure is as follows: 4. What is the Lewis dot diagram for calcium? Calcium is in Group 2 (also known as Group II or 2A), which means it has two valence electrons. To draw the Lewis structure for Calcium, draw two "dots" or valance electrons around the element symbol (Ca). Calcium oxide's ionic formula is simply CaO. What is the Lewis dot symbol? The molecular formula for calcium chloride is CaCl2. Why do you put a Cl at the end of an electron dot diagram? Put a Cl at the end.Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in These diagrams are used as a shorthand notation to show the number of valence electrons in an atom.
Dot diagram for calcium. Draw the dot diagrams for calcium and oxygen. 5. Based on the dot diagram for the atoms in Exercise 4, identify what you expect the charges on calcium ions and oxide ions to be when they form compounds. Explain your answer. 6. Draw the dot diagram for an atom of carbon. 7. Identify how carbon can attain the stable noble gas configuration of 8 ... Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in These diagrams are used as a shorthand notation to show the number of valence electrons in an atom. More complicated versions can be used to show the bond between different atoms in a molecule. The molecular formula for calcium chloride is CaCl2. Gas chromatography was used to measure the maternal and fetal plasma inorganic fluoride values at term in 91 women. They were assigned to one of four groups: group A were untreated controls; group B received a single daily dose of 1.5 mg of fluoride (as calcium fluoride) during the final trimester of pregnancy; group C was given a single dose of 1.5 mg of fluoride (as sodium fluoride) and ... Calcium phosphide appears as red-brown crystals to gray granular lumps. It reacts with water to form calcium hydroxide and phosphine, a flammable poisonous gas. Phosphine will normally ignite spontaneously in contact with air. If there is an excess of water this fire of phosphine will not normally ignite surrounding combustible material.
Calcium oxide electron dot diagram. The formula for silicon oxide is sio 2. When adding calcium and oxygen together they for calcium oxide or cao and calciums 2 valence electrons join with oxygens 6 to fill the lewis structure. The outer electron of the sodium atom 281 is transferred to the outer shell of the chlorine atom 287 giving it a ... What is the electron dot diagram for calcium? After that I draw the Lewis dot structure for Calcium (Ca). Note: Calcium is in Group 2 (sometimes called Group II or 2A). Since it is in Group 2 it will have 2 valence electrons. When you draw the Lewis structure for Calcium you'll put two "dots" or valance electrons around the element symbol (Ca). Topic: Lewis Dot Diagrams for Ionic Compounds. Do Now: Identify ionic compounds. CO. 2 MgCl. 2 NH. 4. ClNaOH. NH. 3 CH. 4 CuSO. 4 HF. Magnesium's outer shell is now empty. Fluorine's outer shell is now full. Lewis Diagrams for Ionic Compounds. NaCl's Lewis structure: ... Lewis Diagram of Calcium Iodide ... what is the lewis dot diagram of calcium the lewis dot diagram for calcium is a visual representation of the element s valence electrons in the outer shell it is symbolized by the abbreviation ca lewis dot structure for calcium ca a step by step explanation of how to draw the lewis dot structure for ca calcium i show you where calcium is on the …
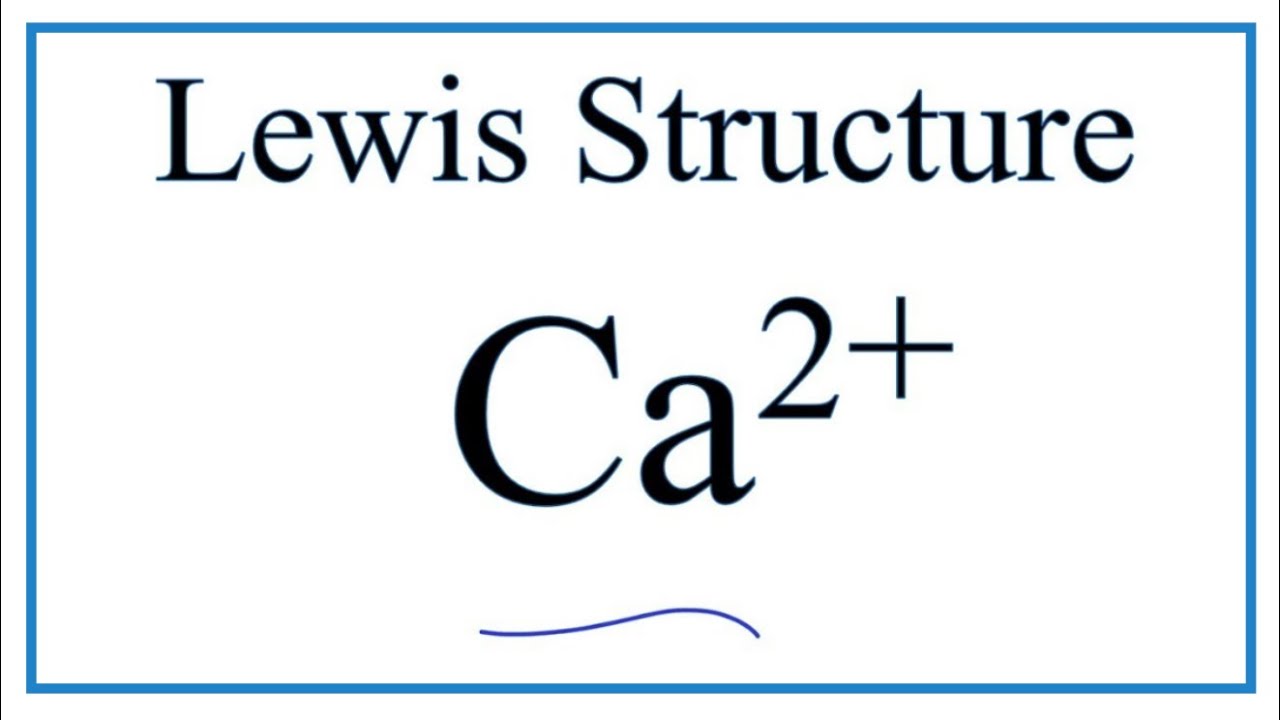
(a) Write electron dot diagram for chlorine (At No. 17) and calcium (At No. 20). Show the formation of calcium chloride by transfer of electrons. (b) Identify the nature of above compound'and explain three physical properties of such compound. For the Ca2+ structure use the periodic table to find the total number of valence electrons for Ca. Once we know how many valence electrons there are in Calc... How do you draw the Electron dot diagram for calcium? First, write Ca on the paper. Next, look at the Periodic Table to find the number of valence electrons. You will see the electron ... Therefore the Calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Video: Calcium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.
Calcium is in Group 2 (sometimes called Group II or 2A). Since it is in Group 2 it will have 2 valence electrons. When you draw the Lewis structure for Calcium you'll put two "dots" or valance electrons around the element symbol (Ca) 7K views View upvotes
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. The dots for a given atom are distributed evenly around the symbol before they are paired.

Dot And Cross Diagram Of Calcium Oxide Showing The Electrons In The Outermost Shells In 2021 Electrons Diagram Chemistry
Read complete answer here. Beside this, what is the electron dot diagram for calcium? Draw the Lewis dot structure for each atom of the compound to show how many valence electrons are present in each atom. For example, the calcium atom in calcium chloride, CaCl2, has two valence electrons, and the chlorine atoms have seven valence electrons each.
Question: Draw a dot- and- cross diagram for magnesium fluoride, showing all electrons. Answer: Dot- and- cross diagram for ionic compound -- magnesium fluoride. Part 3: Dot- and- cross diagrams for simple molecules (covalent molecules). Recap - Covalent molecules are made up of two of more atoms of non- metals.
How do you draw a Lewis dot diagram for calcium? Note: Calcium is in Group 2 (sometimes called Group II or 2A). Since it is in Group 2 it will have 2 valence electrons. When you draw the Lewis structure for Calcium you'll put two "dots" or valance electrons around the element symbol (Ca). Click to see full answer.
Electron Orbital Diagram. lewis dot structure for calcium ca a step by step explanation of how to draw the lewis dot structure for ca calcium i show you where calcium is on the periodic table and how to. Electron Configuration Worksheet Answers Part A Worksheets for. calcium cation calcium cation is a fibrin stabilizing plasma enzyme ...
The molecular formula for calcium chloride is CaCl2. Why do you put a Cl at the end of an electron dot diagram? Put a Cl at the end.Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in These diagrams are used as a shorthand notation to show the number of valence electrons in an atom.
What is the Lewis dot diagram for calcium? Calcium is in Group 2 (also known as Group II or 2A), which means it has two valence electrons. To draw the Lewis structure for Calcium, draw two "dots" or valance electrons around the element symbol (Ca). Calcium oxide's ionic formula is simply CaO. What is the Lewis dot symbol?
The Lewis structure shows the calcium with no dots (electrons), and the chlorine ions with a complete octet. Notice the placement of the charge notation on the ions. 3. The Ca and Cls are near each other, but the two dots (electrons) from each Cl should not be interpreted as a covalent bond. The final Lewis dot structure is as follows: 4.

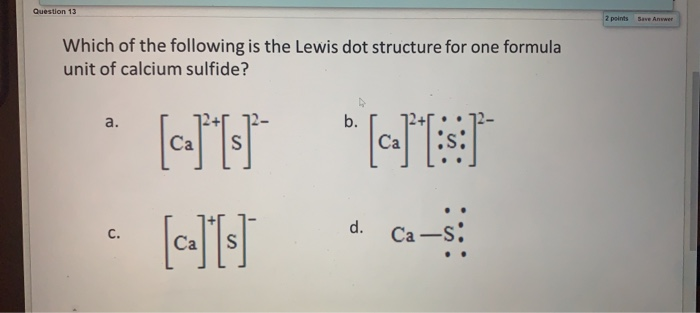
In A Molecule Of Calcium Sulfide Calcium Has Two Valence Electron Bonds And A Sulfur Atom Has Six Brainly Com

Antacid Tablets Commonly Contain Calcium Carbonate And Or Magnesium Hydroxide Draw The Lewis Structures For Calcium Carbonate And Magnesium Hydroxide Study Com

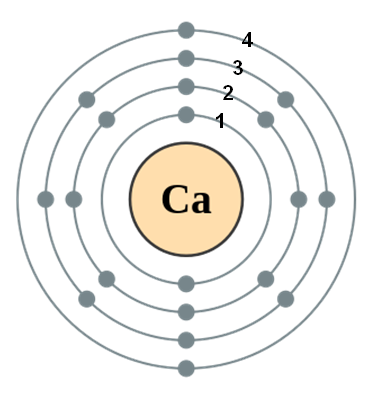
1 Draw The Bohr Rutherford Diagram For Calcium Showing The Nuclear Contents And Correct Placement Of Electrons Homeworklib

Electron Dot Formulas Chemistry 7 C Lesson Objectives Draw Electron Dot Formulas Ionic Compounds Covalent Compounds Electron Dot Formulas Ppt Download
Solved Draw Calcium Ion Lewis Dot Diagram Draw A Calcium Ion Bohr Rutherford Diagram How Many Electrons Does Calcium Lose When It Ionizes What Is Course Hero

How Many Dots Are Shown In The Electron Dot Diagram For Calcium In Group Ii And Period 4 With 20 Brainly Com






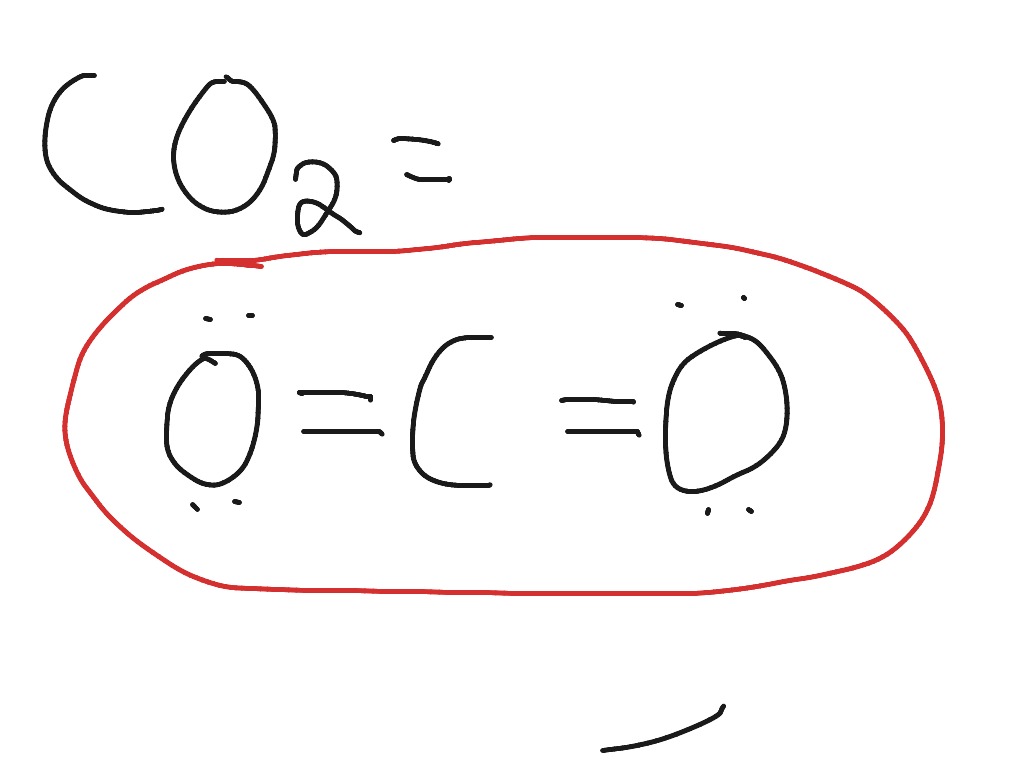

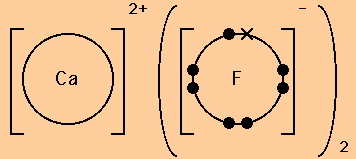







Comments
Post a Comment