40 h2 electron dot diagram
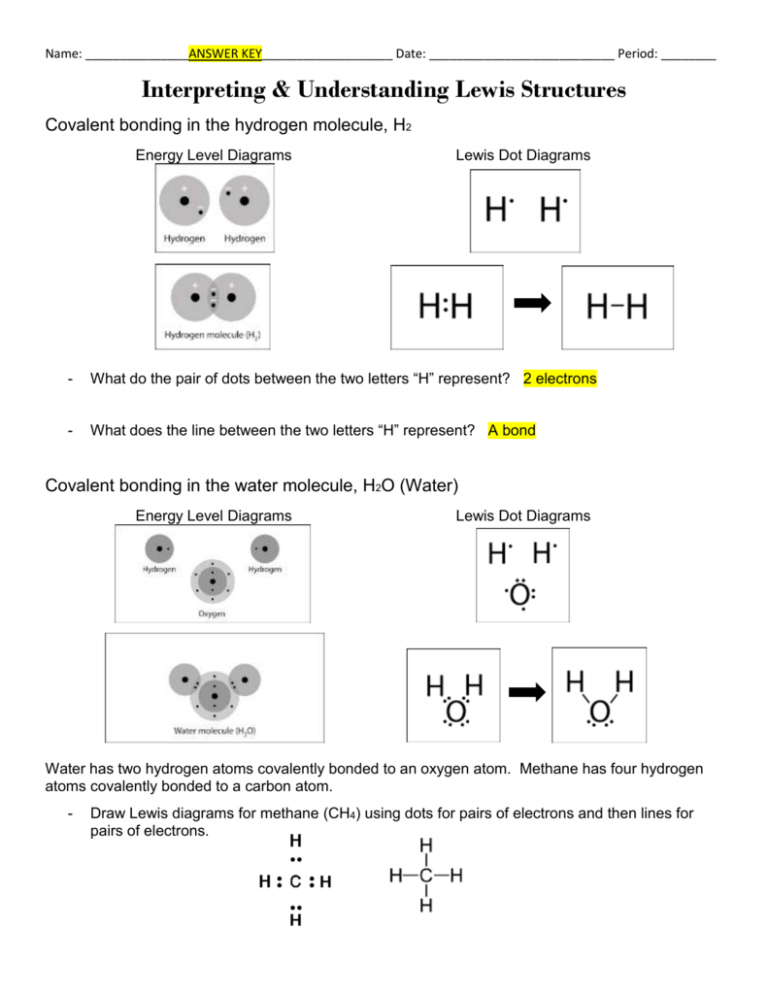
The Lewis Dot Structure for H 2:. Molecular hydrogen (H 2) is a flammable gas.The Lewis structure is quite simple to draw, and shows how each H atom contributes it's valence electron in making the ... What would be the electron dot structure of a molecule of sulphur which is made up of 8 atoms of sulphur? (Hint- The eight atoms of sulphur are joined together in form of a ring.) Medium. View solution. Write the electron-dot structures for (i) ethane, (ii) ethene. Medium.
A step-by-step explanation of how to draw the CaH2 Lewis Dot Structure.For CaH2 we have an ionic compound and we need to take that into account when we draw ...
H2 electron dot diagram
Lewis dot structure of H 2 CO. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. H:1x2=2 C:4 O Total= May 03, · May 6, - Uploaded by Wayne Breslyn. For the CH2O Lewis structure, calculate the total number of valence electrons for the CH2O molecule. The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. 32846. Table of contents. Contributors and Attributions. Describe the hydrogen molecule in light of the following: H − H. H: H. Valence bond theory of H 2. Molecular orbital theory of H 2. Electron configuration of molecules.
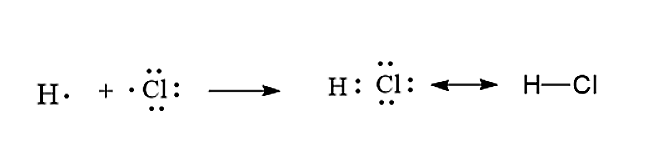
H2 electron dot diagram. We can use Lewis dot formulas to show covalent bond formation. 1. H. 2 molecule. +. H. H. H H.. or H2. H. Cl . Resonance is a flawed method of representing. Draw a Lewis electron dot diagram for an atom or a monatomic ion. of how valence electrons interact, a simple way of representing those valence electrons . What is the dot structure of H2? On the left is a single hydrogen atom with one electron. On the right is an H2 molecule showing the electron cloud overlap. The shared pair of electrons is shown as two dots in between the two H symbols (H:H). This is called a single covalent bond, when two atoms are joined by the sharing of one pair of electrons. H2S Lewis Structure. The lewis structure of H2S is as below. Now to understand this we need to know the steps to draw a lewis structure at first. First and foremost it is important to determine how many valence electrons are present in the compound. In this compound valence electron will be as following: Hydrogen valence electron = 1. 2 ... -H2. NH3. Which compound contains a triple bond?-ammonia (NH3)-methane (CH4)-chlorine (Cl2)-acetylene (C2H2) acetylene (C2H2) In an electron dot diagram of propane (C3H8), how many double bonds are present?-three-none-one-two. none. What is the molecular shape of silicon tetrabromide?-tetrahedron-bent triatomic-pyramidal
The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons in oxygen are in pairs at the bottom. The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom. Answer (1 of 3): A Lewis structure is a diagram that shows the bonding between the atoms of a molecule and any possible lone pairs of electrons. To ensure stability, most atoms require an octet - that is, 8 electrons in their outermost electron shell. Hydrogen, however, is an exception. An atom ... The Lewis diagram of hydrogen is the most simplified 'dot and cross' style of electronic diagram for the covalently bonded hydrogen molecule. The hydrogen molecule is held together by the strong hydrogen-hydrogen single covalent bond H-H (displayed formula, a simple representation of the single bond but no electronic structure detail). This is the electron dot diagram for the H2 molecule: H:H Using numbers, complete the following statement. In the hydrogen molecule, there are bonding electron pair (s) and lone electron pair (s). Check Answ. This is the electron dot diagram for the F2 molecule: Using numbers, complete the following statement In the fluorine molecule, there are ...
A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Diatomic Hydrogen).Note that Diatomic Hydrogen is often called Molecular Hydrogen or ju... Lewis Structures for O2. Step-by-step tutorial for drawing the Lewis Structure for O2. The covalent bond in an oxygen molecule, O 2 (oxygen gas) is non-polar - electrons are shared equally. Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen. Draw The Electron Dot Structure Of H2. Draw the electron dot structure of H2. The electron dot structure or Lewis structure of H2 (H-H) is given below: Was this answer helpful? 0 (0) (0) (0) Choose An Option That Best Describes Your Problem. Answer not in Detail. Incomplete Answer. Answer Incorrect. Others. H2o2 Dot Diagram. The chemical name for H2 O2 is hydrogen peroxide. Its Lewis structure shows us where the valence electrons are located in the molecule, which can aid us in. Count the number of electrons, add single bonds between the atoms, using two electrons per bond, arrange the remaining electrons around the.
Nov 01, 2021 · 13+ H2 Lewis Structure. You have a total of 8 valence electrons available to fill the octets of oxygen and hydrogen. All valence electrons of the atoms in lewis structures must be shown. H2S Lewis Structure – How to Draw the Dot Structure for … from i.ytimg.com. Most lewis structures you encounter will be covalent bonds.
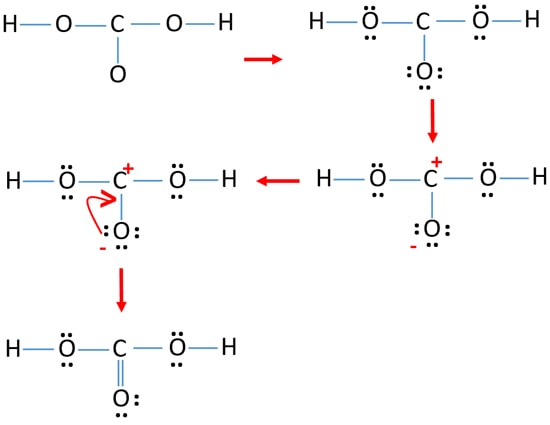
Solved Examples on Electron Dot Formula. Q.1: Explain the Electron Dot Formula of. Solution: The central atom of this molecule is the carbon atom. Oxygen contains 6 valence electrons with 2 lone pairs. Then, it is bonded to only one carbon atom with a double bond. Carbon contains four valence electrons, therefore giving zero lone pairs.
Lewis dot structure of hydrogen fluoride. Source: cdn.instructables.com. Lewis dot structure practice problems (with answers and explanation). Source: i.ytimg.com. Atomic or orbit structural diagram. Source: showme0-9071.kxcdn.com. On the right is an h2 molecule showing the electron cloud overlap. Source: upload.wikimedia.org
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
/EXPL THER/ Hydrogen-rich saline (HRS) is a novel protection against various oxidative disorders and almost all types of inflammation.Moreover, its toxicity and side effects are rarely reported. We sought to clarify the protective effect of HRS against the oxygen-induced retinopathy (OIR) in C57BL/6 J model.The OIR in the HRS treated mice and the untreated controls were systematically compared.
Lewis Dot Structure for Hydrogen(H,H2) Hello,today I am going to draw the lewis dot structure for hydrogen in just two steps. Step-1: To draw the lewis Dot structure of hydrogen, we have to find out the valence electrons of hydrogen first.We express valence electrons as dots in lewis dot structure.
Sep 24, · Lewis dot structures for a nonpolar molecule must show equal electron distribution throughout the molecule, thus having no dipole. For a simple example: H2 is nonpolar. So H-H would be represented with H (two dots)H. 2. Fluorine has 7/8 valence electrons, therefore F is surrounded by 3 sets of pairs of dots and 1 single dot on one ...
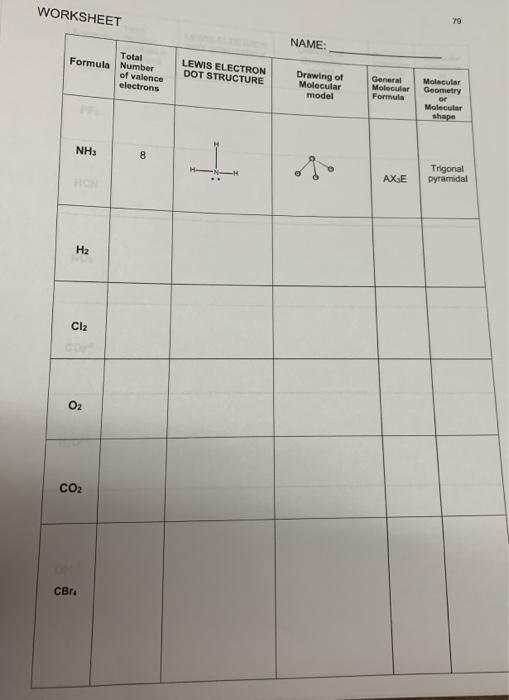
To use dot diagrams to show how electrons can be transferred or shared to form bonds. ICl NH3 MgS H2O PCl3. MgCl2 CO2 NaCl Na2O CH4 CHCl3 O2 H2 HCl OH- Directions: Draw electron dot diagrams for each element in the compounds above. Draw only the valence electrons. Put one electron on each of the "4 sides" first, before pairing them up.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
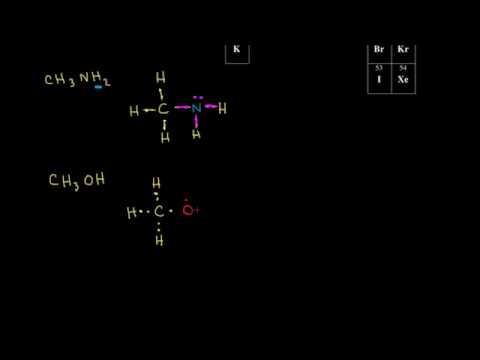
Lewis dot structure of H?? what is the lewis dot structure of H2O2??? Follow. 2 answers 2. Report Abuse. draw the diagram of H2, the diagram of O2 then connect them where it should be connected. IamCat · 1 decade ago. schematron.org: Resolved. Mar 11, · In the chemical formula of hydrogen peroxide(H2O2) why does both the 2 does not get ...
Let's do the Lewis structure for H2, Hydrogen gas. It's a quite explosive gas, so please don't fill your blimp up with it. Let's look at the periodic table. Hydrogen is in group 1, that means it has 1 valence electron. But we have 2 Hydrogen atoms, so let's multiply that by 2, for a total of 2 valence electrons.
Lewis electron dot-diagrams for CO2 and SO2 are given above. the molecular geometry and polarity of the two substances are. ... because pure H2 is a hazardous substance, safer and more cost effective techniques to store it as a solid for shipping purposes has been developed. One such method is the reaction represented above, which occurs at 200C.
Check the Formal Charges to make sure you have the best Lewis Structure. Explain How Examples: SO 4 2-, N 2 O, XeO 3; Notable Exceptions to the Octet Rule. H only needs 2 valence electrons. Be and B don't need 8 valence electrons. S and P sometimes have more than 8 val. Electrons.
Lewis Structure Hydrogen Atom Electron Png Clipart Angle Atom Bicarbonate Black Black And White Free Png
A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Hydrogen gas).For the H2 structure use the periodic table to find the total number of v...
32846. Table of contents. Contributors and Attributions. Describe the hydrogen molecule in light of the following: H − H. H: H. Valence bond theory of H 2. Molecular orbital theory of H 2. Electron configuration of molecules.
The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol.
Lewis dot structure of H 2 CO. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. H:1x2=2 C:4 O Total= May 03, · May 6, - Uploaded by Wayne Breslyn. For the CH2O Lewis structure, calculate the total number of valence electrons for the CH2O molecule.

In The Space Provided Below Draw Electron Dot Diagrams For The Following Molecules Hydrogen H2 Brainly Com





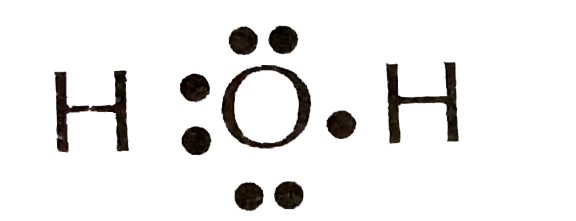






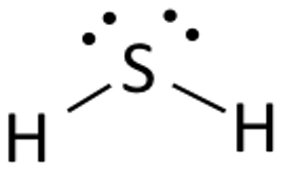









/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
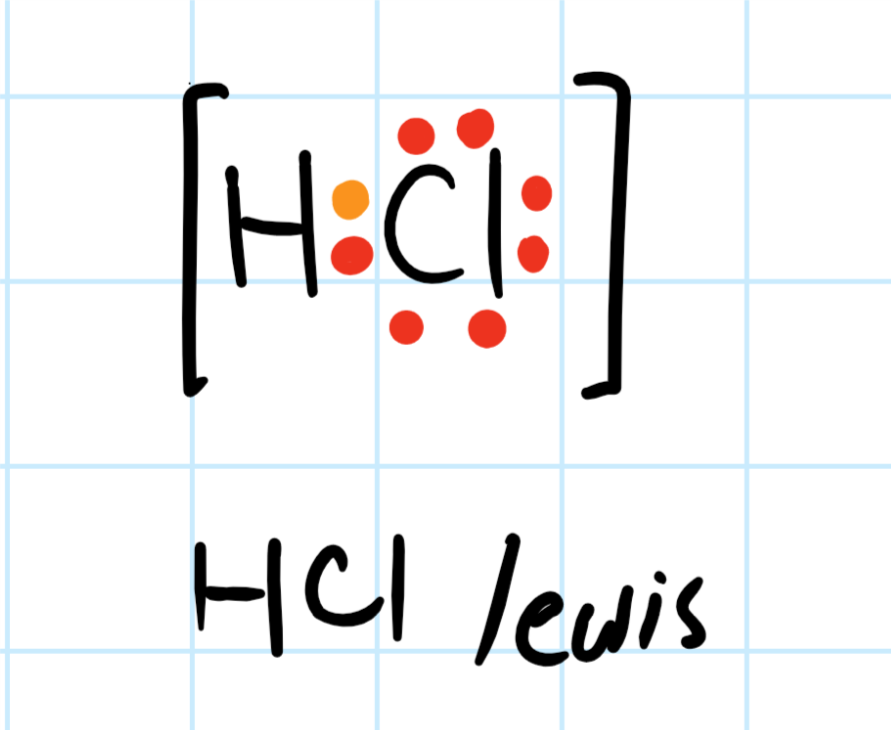

Comments
Post a Comment