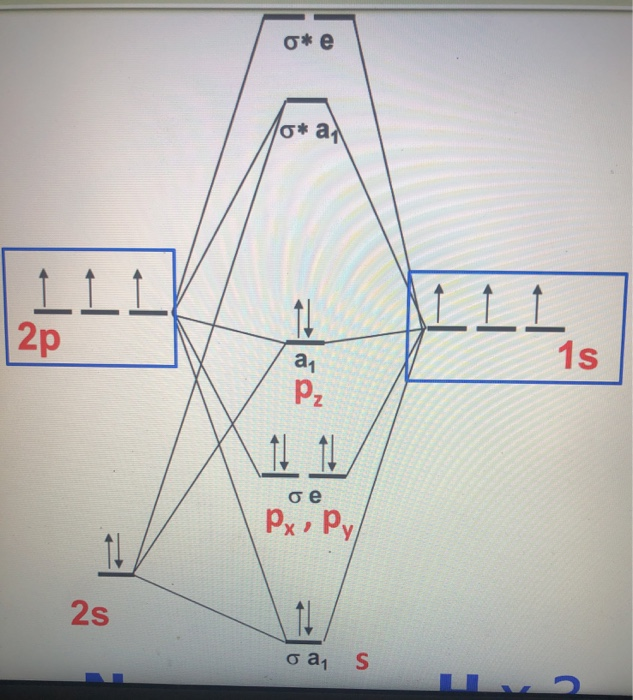
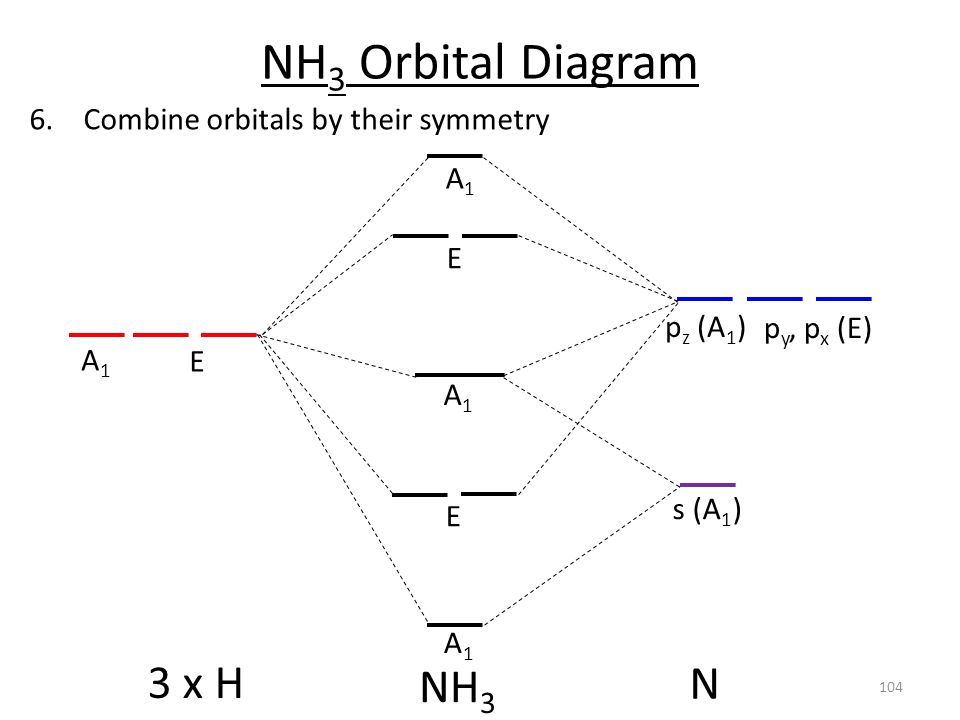
42 nh3 mo diagram
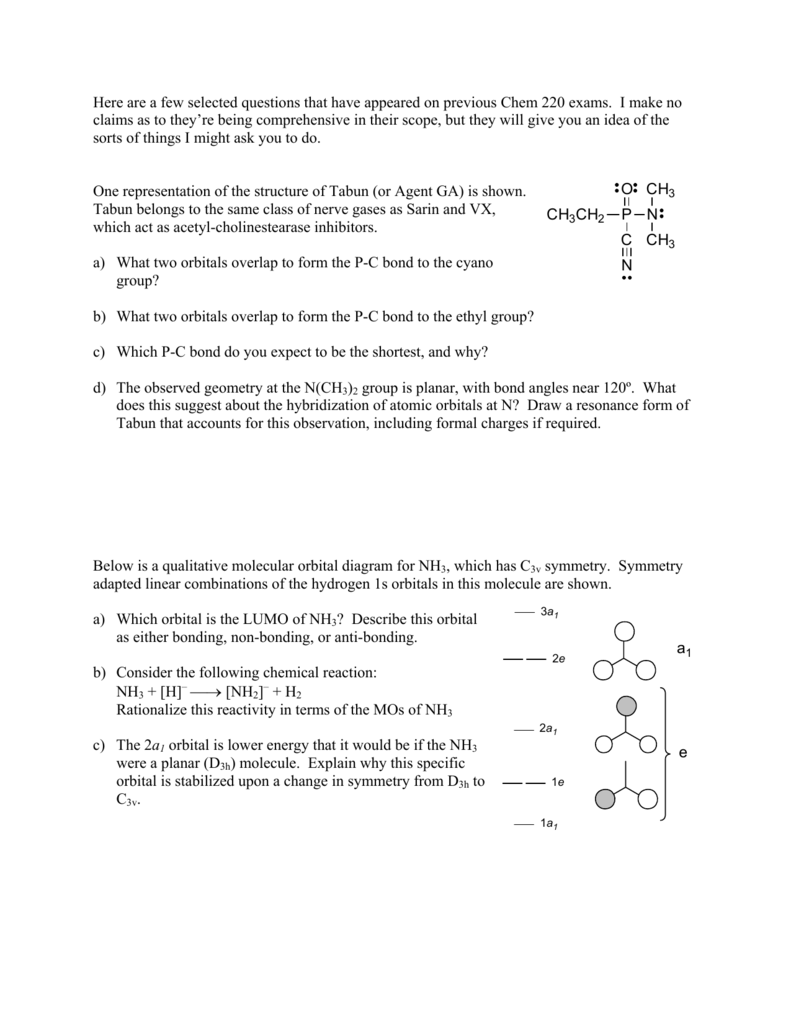
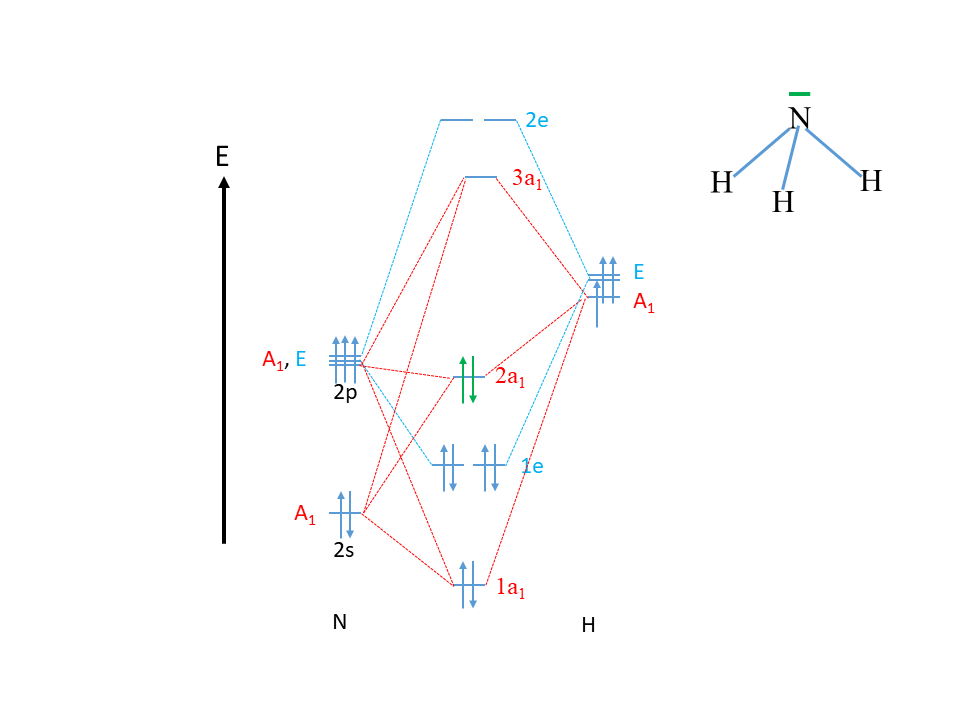
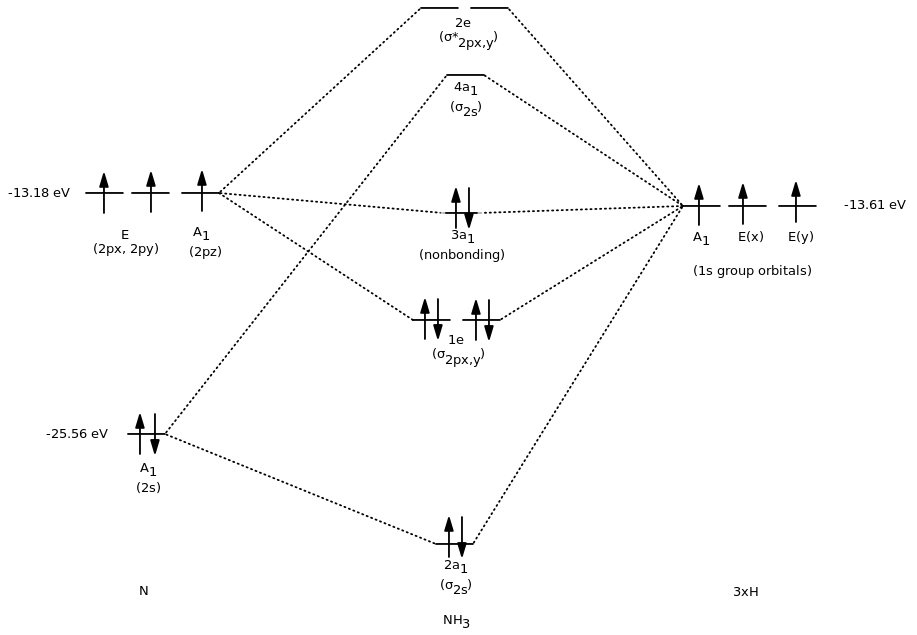
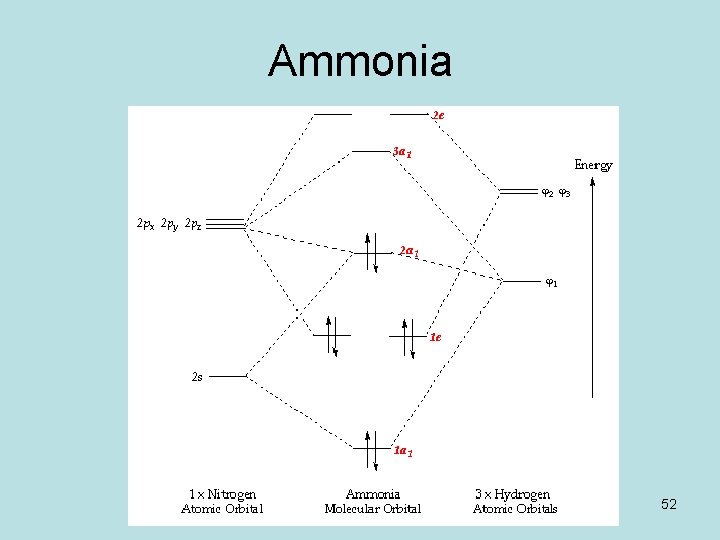
Problem: The results of a molecular orbital calculation for NH3 are shown here.Examine each of the orbitals and classify them as bonding, antibonding, or nonbonding. Assign the correct number of electrons to the energy diagram. Chem 302. The Molecular Orbitals of Ammonia. Determing the electronic structure of ammonia will introduce the new ideas of degenerate orbitals and degenerate axes. It is important to understand these concepts because of the large number of molecules that have point groups such as C 3v or D 3h. To determine the MO's of ammonia.
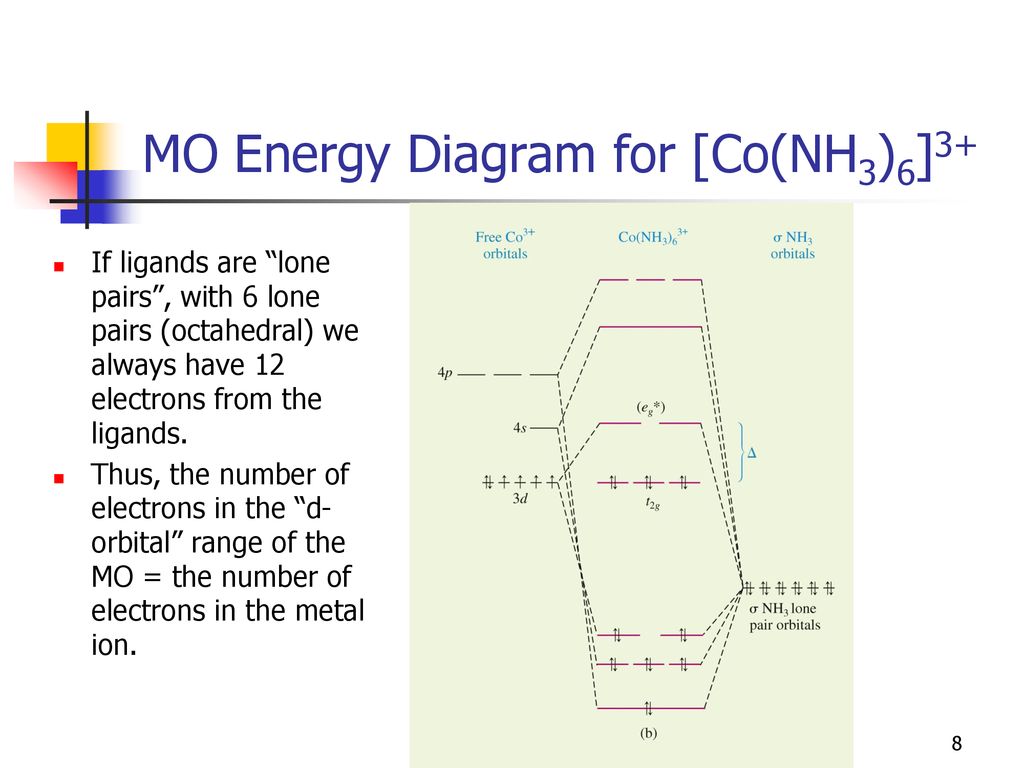
I was considering the MO diagram for [Co (NH3)5Cl]2+, and figured it'd be as follows: 5 sigma interactions from the NH3 ligands; by reducing a reducible representation one obtains 2A1 + B1 + E. 2 pi interactions (pi donation from Cl- px and pz orbitals), which have symmetry E. And the cation atomic orbitals, which we just read off the ...

Nh3 mo diagram
Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.facebook.com/l.php?u=https%3A%2F%2Ffb.me%2F... For reference we have kept the d-orbital labels on this diagram. Note the non-bonding d-orbitals. * MO Energy Diagram for [Co(NH3)6]3+ If ligands are "lone pairs", with 6 lone pairs (octahedral) we always have 12 electrons from the ligands. Thus, the number of electrons in the "d-orbital" range of the MO = the number of electrons in the metal ion. To develop a MO scheme for NH3 assume that only the 2s and2p orbitals of ... for constructing the MO diagram we will assume that px orbitals are used, ...30 pages
Nh3 mo diagram. * H3+ * BeH2 * Correlation diagram for MH2 M < HMH Be 180° B 131 C 136 N 103 O 105 * Bonding MO's in H2O * NH3 Use triangular H3 MO's from above as SALC's of the H ligand orbitals. Must relabel to conform with lower symmetry pt group C3v. They become a1 and e. Combine with N valence orbitals with same symmetry. Nh3 Mo Diagram. Here are a number of highest rated Nh3 Mo Diagram pictures on internet. We identified it from obedient source. Its submitted by direction in the best field. We receive this kind of Nh3 Mo Diagram graphic could possibly be the most trending subject once we portion it in google help or facebook. MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions. The s orbitals for the 3 hydrogens are used to set up the sigma and anti bonding combinations of N sp 3 orbitals and the H 1s orbitals.Introduction to Molecular Orbital TheoryWhat is the hybridization of NH_3? | Socratic Chemistry questions and answers. Question 1 3.5 Points On the basis of Ligand Field Theory (LFT), draw the MO diagram and write the electronic configuration of the complex [Fe (NH3)6]3+. Indicate weather it is high spin or low spin complex. Use the editor to format your answer. The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions. The s orbitals for the 3 hydrogens are used to set up the sigma and anti bonding combinations of N sp 3 orbitals and the H 1s orbitals. Derive the molecular orbital diagrams for linear and bent H 2 O. I need some help drawing a NH3 molecular orbital. I've made a drawing off how it should look like (but it looks ugly, the proportions are wrong and and and). It would be nice if you could help me drawing such a diagram. All the text used in on the sketch should be used, but please all in black, colors was only used to show how it should look ...
Construct an approximate molecular orbital energy diagram for a hypothetical planar form of NH3. From a consideration of the atomic energy levels, place the N and H3 orbitals on either side of a molecular orbital energy-level diagram. Then use your judgement about the effect of bonding and anti bonding interactions and energies of the parent ... The MO energy diagram for NH3 is shown in Figure 3. The energy. As can be seen from the energy diagram - four of the molecular orbitals occur as Ammonia has two pairs of degenerate orbitals, one bonding and one.MO Diagram for Triangular H 3 A fragment approach to deriving molecular orbitals Inorganic Chemistry. H.O Fig. 3. Solubility isotherm of the NH3-Mo03-H,0 system at 25 . The phase diagram developed for the polymolybdate region of the NH3o0320 system does not always agree with earlier reports on this system. The solubility isotherm for APM at 25 C agrees well with that of Foote and Bradley at high NHa concentrations. Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
Making the MO diagram. SYMMETRY CONSIDERATIONS Ammonia belongs to what is called the C_(3v) "point group", because it has a principal, C_3 rotation axis, and because it has a vertical mirror plane sigma_v. A hatC_3 operation is when you rotate 360^@/3 = 120^@ and achieve the original molecule back.
CHE 422 Inorganic Chemistry. Molecular orbital energy level diagram of NH3 and Frontier Orbitals (highest occupied molecular orbital HOMO and lowest unoccup...
Stable electronic configurations: MO Energy Level Diagrams Reviewed . Electron count preference . Electron count and Oxidation States Stable electronic configurations: MO Energy Level Diagrams Reviewed Electron count preference Electron count and Oxidation States Ligands • Carbon Monoxide • Phosphines
Nh3 Molecular Orbital Diagram. Ammonia is a trigonal pyramidal molecule with three pendant hydrogen atoms. The MO energy diagram for NH3 is shown in Figure 3. However for the species with more than 16 electrons in Table 8 the π u antibonding molecular orbital will be occupied and the linear geometry will have a higher energy than the bend ...
For the octahedral complex ion [CO(NH3)6]3+ a) Draw a molecular orbital (MO) energy level diagram. (2 Marks) b) Calculate the number of unparied electrons. (0.5 Mark) c) Indicate weather it is high spin or low spin complex. (0.5 Mark) 2. Breifly explain the synergic bond in the Cr(CO)6. Illustrate your answer with drawing, 12 Marks) (B) (5
A further example is given using the NH3 MO diagram. Here, they calculate the bond order as 3, ignoring the fact that the NH3 a1 orbital is weakly bonding. If we were to in theory calculate the MO diagram for NH3 in a trigonal planar geometry (same as for BH3), we would also get the bond order as 3.
The energy level diagrams are shown in Figures 1, 2, 3a, b. It should be pointed out that the dotted lines connecting the a.o.'s to the m.o.'s represent a one electron partici- pation (or population) of greater than 10%. ... Energy levels for [Ni(NH3)6]:+ Fu =1.3. Shupack I Molecular Orbital Theory for Metal-Ammine Complexes \ a, e t,. 442 ...
The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions. The s orbitals for the 3 hydrogens are used to set up the sigma and anti bonding combinations of N sp 3 orbitals and the H 1s orbitals.
Molecular orbital diagram of ammonia (NH3) molecule The molecular orbital diagram is a diagrammatic representation of how chemical bonding is taking place within the molecules. In the case of ammonia (NH3), the molecular orbital diagram helps with understanding how sigma bonds are formed.
Molecular Orbital Diagrams • review a few MO concepts • generate MO for XH 2, H 2 O, SF 6 Formation of a bond occurs when electron density collects between the two bonded nuclei (ie., Ψ2 MO = large in the region of space between nuclei) Spin-pairing Bonding Molecular Orbital Antibonding Molecular Orbital
In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction. No Metal- Ligand -bonding ( bonding only) Let's take [Co(NH3)6]3+ as an example. Using the LGO method, one can construct a qualitative MO diagram for bonding in a [ML6]n+ complex.
To develop a MO scheme for NH3 assume that only the 2s and2p orbitals of ... for constructing the MO diagram we will assume that px orbitals are used, ...30 pages
For reference we have kept the d-orbital labels on this diagram. Note the non-bonding d-orbitals. * MO Energy Diagram for [Co(NH3)6]3+ If ligands are "lone pairs", with 6 lone pairs (octahedral) we always have 12 electrons from the ligands. Thus, the number of electrons in the "d-orbital" range of the MO = the number of electrons in the metal ion.
Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.facebook.com/l.php?u=https%3A%2F%2Ffb.me%2F...
![PDF] A brief introduction to molecular orbital theory of ...](https://d3i71xaburhd42.cloudfront.net/a9dad92b9b44a403edfe334943a87a50cb998c24/3-Figure6-1.png)



















Comments
Post a Comment