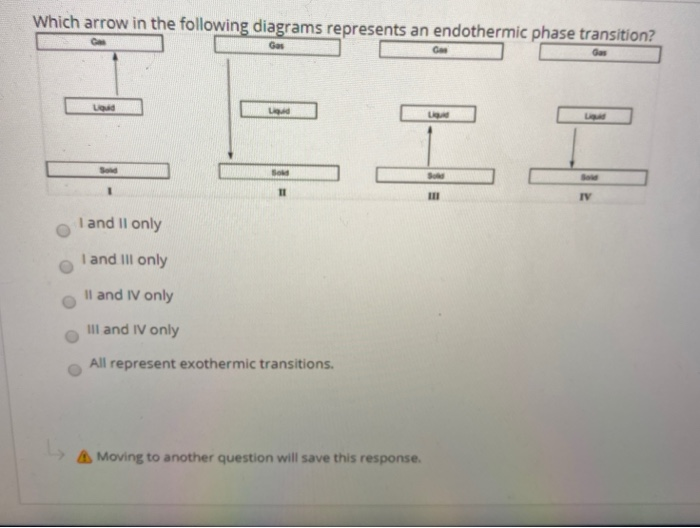
40 phase change diagram endothermic exothermic
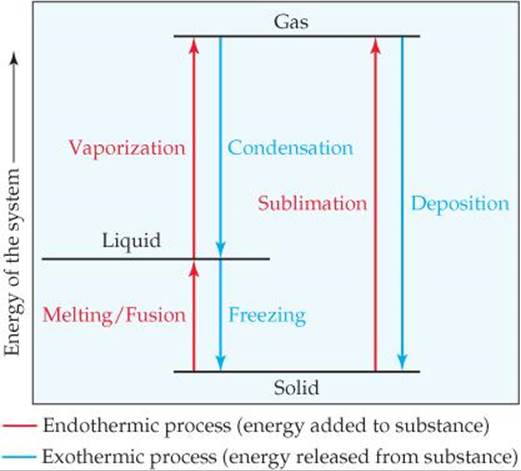
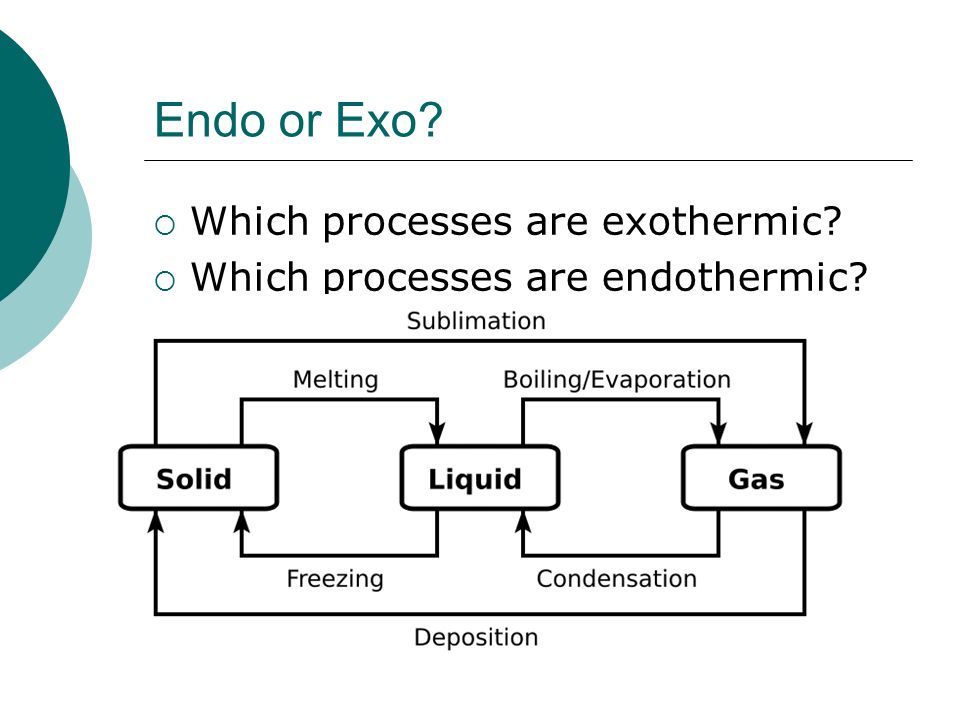
Phase Change/Diagram Practice Flashcards | Quizlet Start studying Phase Change/Diagram Practice. Learn vocabulary, terms, and more with flashcards, games, and other study tools. How do you know if a phase change is endothermic or ... What phase change is endothermic? Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system.
Endothermic vs. exothermic reactions (article) | Khan Academy Phase diagrams. Enthalpy. Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity. Gibbs free energy example. More rigorous Gibbs free energy / spontaneity relationship. A look at a seductive but wrong Gibbs spontaneity proof. Endothermic vs. exothermic reactions.

Phase change diagram endothermic exothermic
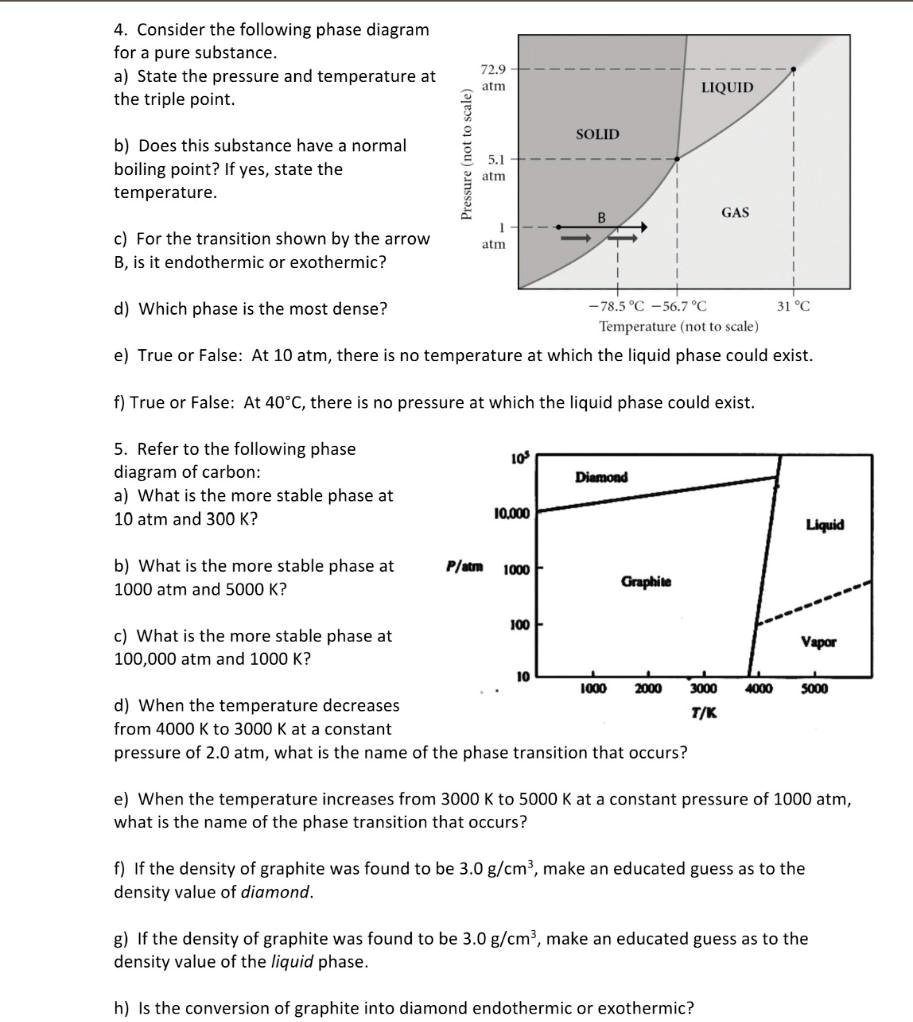
PDF Lecture Notes 2: Physical Equilibria - Phase Diagrams This diagram shows the regions of stability of different phases as a function of temperature and pressure. The interfaces between these regions will be the phase transition lines. The phase diagram for CO 2is shown below. Things to notice on the diagram. 1. Given a pressure and a temperature you can find the stable phase (gas, solid, or liquid) 2. 10.3: Energy and Phase Changes - Chemistry LibreTexts Transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are exothermic and release energy. The energies absorbed in the endothermic steps are identical in magnitude to the energies released in the exothermic steps for the same transition. ΔH Fusion = -ΔH Freezing Phase Changes, Diagrams - CourseNotes Find the enthalpy change as 10 g of a liquid at 70° C goes to a gas at 100° C Given: liquid boils at 90° C specific heat of liquid = 1.0 J/g-K specific heat of gas = 0.3 J/g-K enthalpy of vaporization = 8.5 J/g enthalpy of heating liquid from 70° C to 90° C = (10) (1.0) (20) = 200 J H vap = (10) (8.5) = 85 J
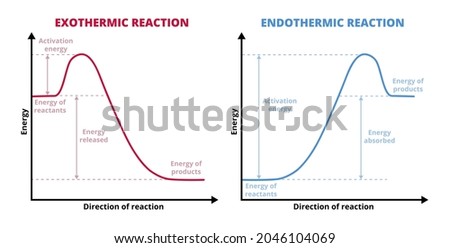
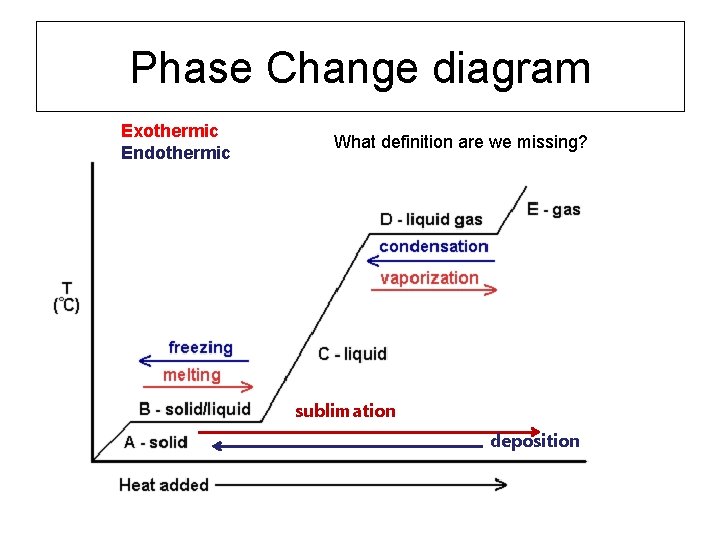
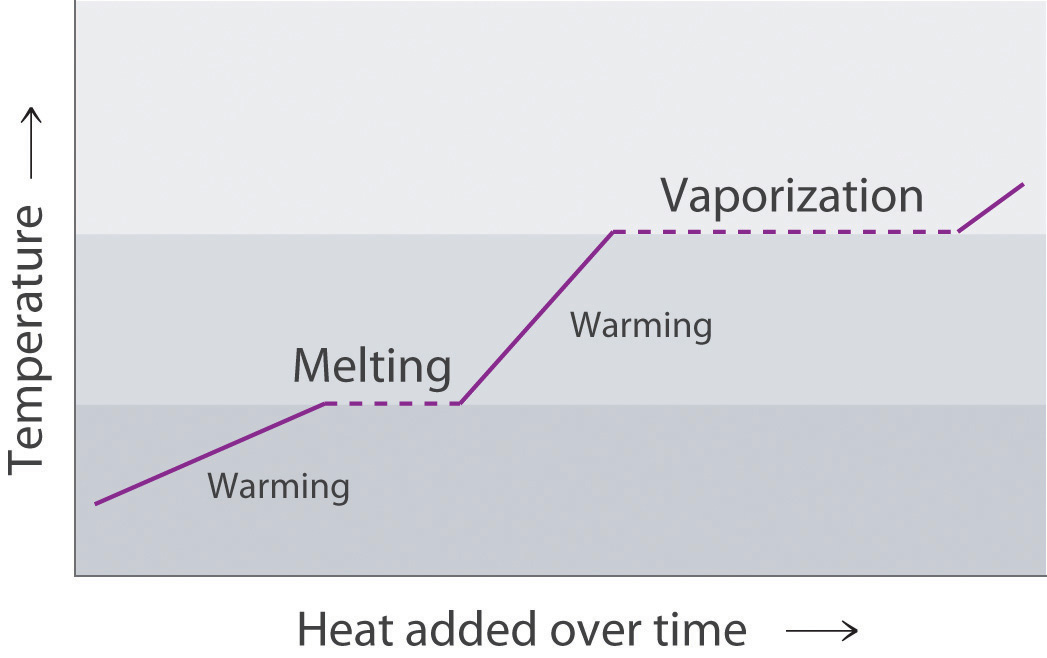
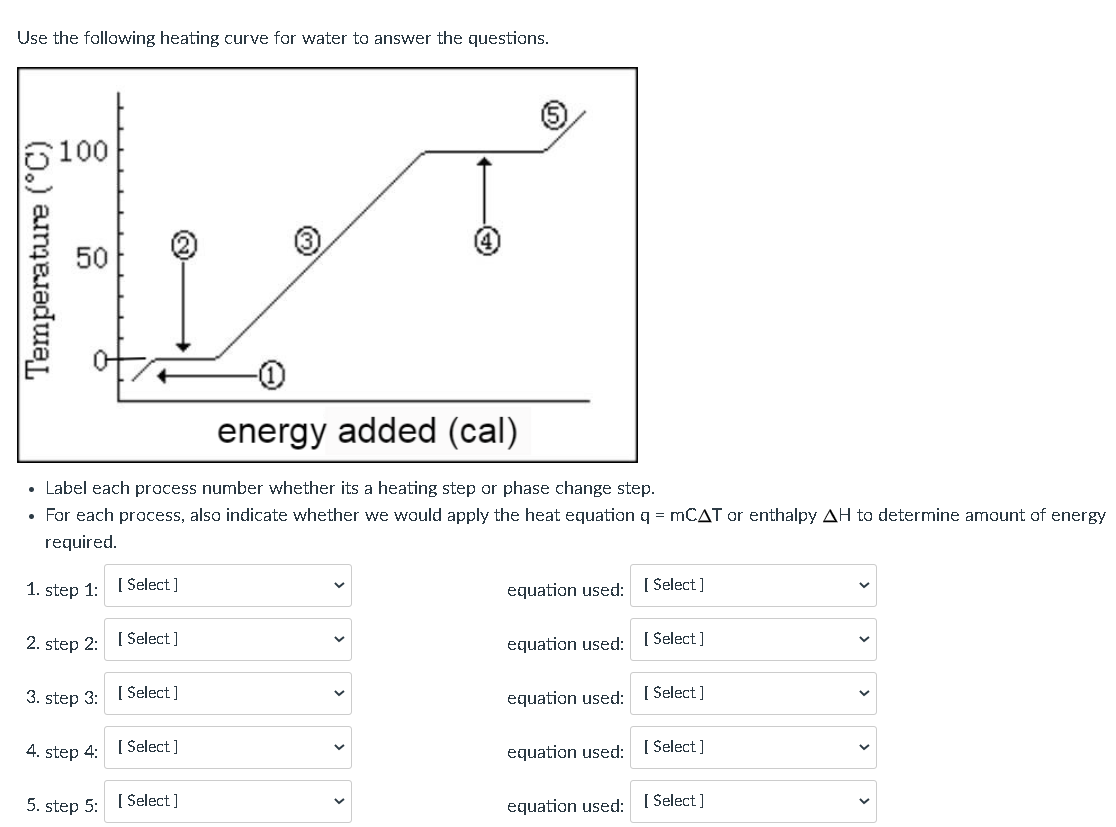
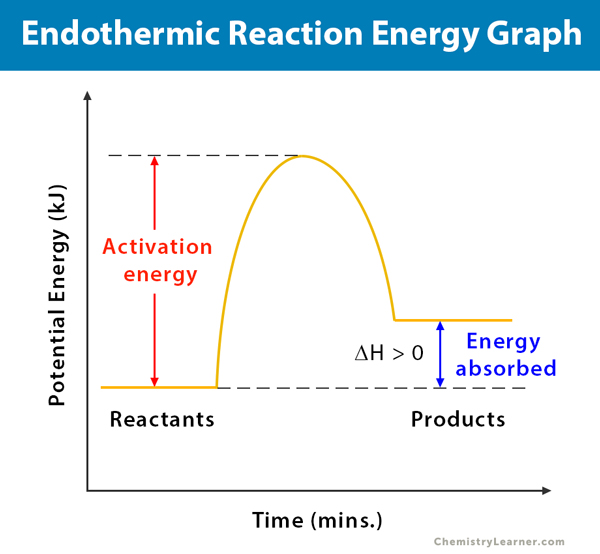
Phase change diagram endothermic exothermic. Endothermic Reaction Coordinate Diagram A reaction is endothermic when the energy of the products is greater than the energy of the reactants. The is for an exothermic reaction. Below is a reaction coordinate diagram for an endothermic reaction. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism. Phase Change - Phase Transition - Nuclear Power In general, sublimation is a phase change of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. Sublimation is an endothermic phase change that occurs at temperatures and pressures below a substance's triple point in its phase diagram. Consider the ice at -10°C and the pressure of 500 Pa. States of Matter / Phase Changes Notes - Google Docs States of Matter Notes State of Matter (name and drawing of particles) Describe Particles Properties (indefinite vs. definite shape/volume) Solid Liquid Gas Plasma Phase Change Diagram Label endothermic phase changes in red and exothermic phase changes... Phase Changes and Energy | 2022 AP Chem Unit 6 Study Guide ... Use q=mcΔT at the slopes (since there is a change in temperature) and Hf (m)/Hv (m) on the plateaus Cooling Curves Cooling curves show the exothermic processes, which is the opposite of the endothermic processes the heating curve shows. Image Courtesy of Schoenherr & Diamantopoulos Chemistry Videos
Physical Equilibria - Chemistry 302 Phase Diagrams. The diagram you mostly find associated with different phases of a substance is the so-called "phase diagram". This diagram shows the regions of stability of different phases as a function of temperature and pressure. The interfaces between these regions will be the phase transition lines. CO 2 Changes of State The phase diagram is another representation of the internal energy of a substance compared with the pressure and temperature of its surroundings. temperature and pressure are inversely related to the process of vaporization pressure has little effect on the melting and freezing of a substance the triple point is a point where all three Solved Be sure to answer all parts. Identify the phase ... Identify the phase change shown in the molecular diagram and indicate whether it is exothermic or endothermic. The phase change shown in this molecular diagram is (select) v The phase change is (select) v Question: Be sure to answer all parts. 3: Phase Changes - Chemistry LibreTexts 3.2: Energy of Phase Changes Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system.
How to Determine if Phase Change is Endothermic or ... 📗 Need help with chemistry? Download 12 Secrets to Acing Chemistry at 💯 If you like my teaching style and are inte... Video Notes-Thermochemistry.doc - Name _ Period _ Video ... (9:34). Draw a potential energy diagram that has 3 steps where the 2 nd step is rate determining and the overall reaction is endothermic. Let's say the 1 st step is endothermic and the 2 nd step is exothermic and the 3 rd step is endothermic. Draw this diagram below. Exothermic, Endothermic, & Chemical Change | Energy ... Identifying Exothermic & Endothermic Reactions. There are two methods for distinguishing between exothermic and endothermic reactions. Monitor temperature change. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When energy is absorbed in an endothermic reaction, the temperature decreases. 10.3 Phase Change Diagrams | Chemistry - Lumen Learning The reciprocal process, freezing, is an exothermic process whose enthalpy change is −6.0 kJ/mol at 0 °C: H2O(l) H2O (s)ΔHfrz = −ΔHfus = −6.01kJ/mol H 2 O ( l) H 2 O ( s) Δ H frz = − Δ H fus = − 6.01 kJ/mol Sublimation and Deposition Figure 6.
Energy Diagrams of Reactions | Fiveable If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings. Phase Changes The basics of exothermic and endothermic reactions is understanding phase changes and which process is taking place. Melting
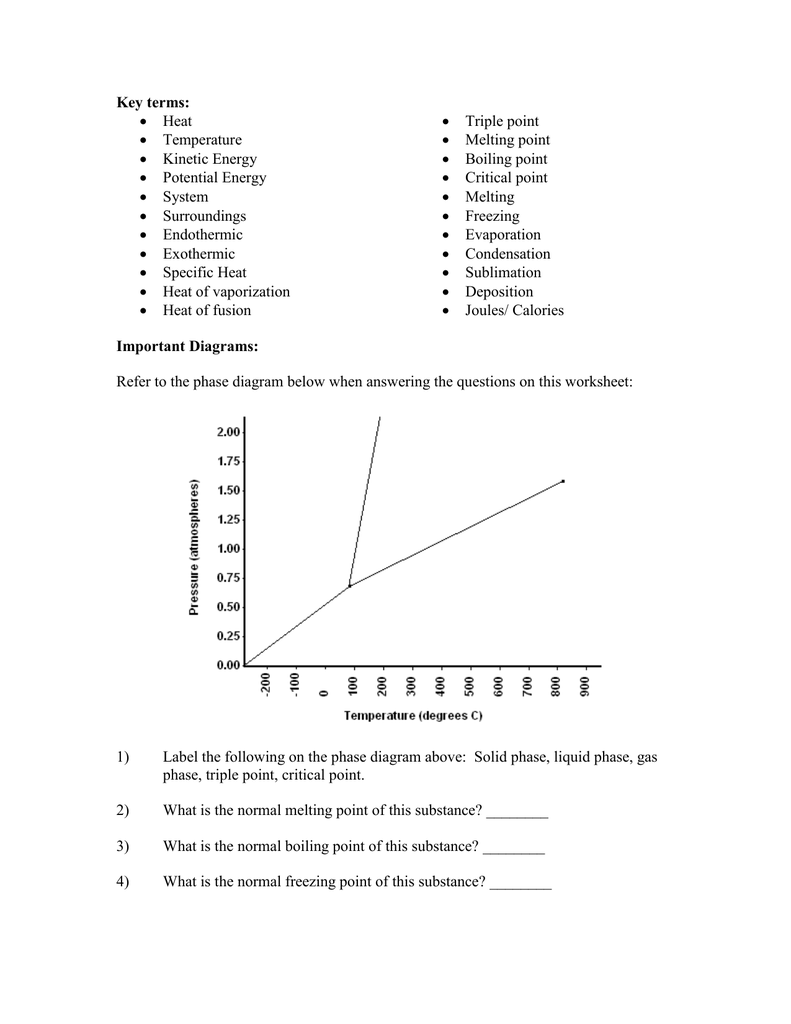
Phase Diagrams | Boundless Chemistry - Lumen Learning Phase Diagram: In this phase diagram, which is typical of most substances, the solid lines represent the phase boundaries.The green line marks the freezing point (or transition from liquid to solid), the blue line marks the boiling point (or transition from liquid to gas), and the red line shows the conditions under which a solid can be converted directly to a gas (and vice-versa).
Phase diagrams (video) | Thermochemistry | Khan Academy In the video here, Sal uses a horizontal line through the phase diagram. But, it doesn't have to be horizontal. Imagine a vertical line through this diagram-- for water, choose 100 degrees C. As long as you are at 100 C, you can change the phase by changing the pressure on the system. This kind of process is called "adiabatic". ( 22 votes)
MCSM Regents Chemistry || Unit #5: Phase Changes Below is a description of the the energy change for the different types of phase changes: Fusion is endothermic Vaporization is endothermic Condensation is exothermic Solidification is exothermic Sublimation is endothermic Deposition is exothermic
Endothermic And Exothermic Reactions Examples ... We take on this kind of Endothermic And Exothermic Reactions Examples graphic could possibly be the most trending subject similar to we allocation it in google help or facebook. We attempt to introduced in this posting back this may be one of astonishing insinuation for any Endothermic And Exothermic Reactions Examples options.
Exothermic and endothermic reactions - Energy changes in ... Exothermic and endothermic reactions When a chemical reaction occurs, energy is transferred to or from the surroundings. There is usually a temperature change. For example, when a bonfire burns it...
Exothermic phase changes? - Answers An endothermic phase change is when the substance absorbs energy from its surroundings (melting, vaporization).In an exothermic phase change the substance releases energy to its surroundings ...
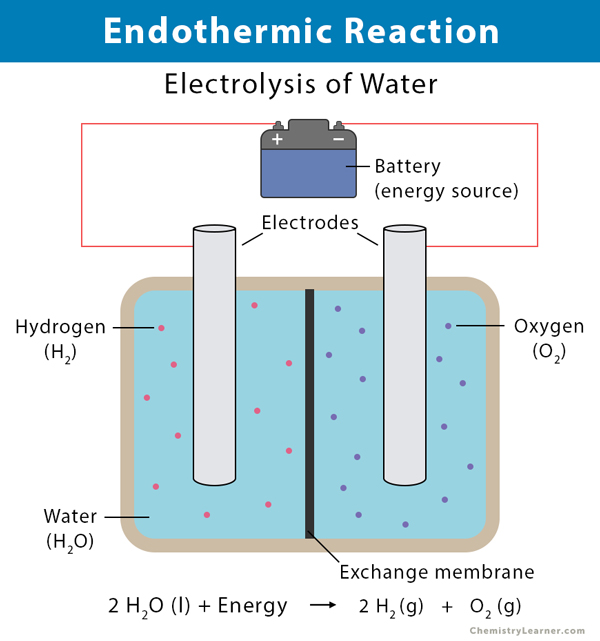
Difference Between Endothermic and Exothermic Reactions ... The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. A few examples of the endothermic process are photosynthesis, evaporating liquids, melting ice, dry ice, alkanes cracking, thermal decomposition, ammonium chloride in water and much more.
Phase Changes, Diagrams - CourseNotes Find the enthalpy change as 10 g of a liquid at 70° C goes to a gas at 100° C Given: liquid boils at 90° C specific heat of liquid = 1.0 J/g-K specific heat of gas = 0.3 J/g-K enthalpy of vaporization = 8.5 J/g enthalpy of heating liquid from 70° C to 90° C = (10) (1.0) (20) = 200 J H vap = (10) (8.5) = 85 J
10.3: Energy and Phase Changes - Chemistry LibreTexts Transitions going up (increased energy) are endothermic and require energy ,while those going down (decreased energy) are exothermic and release energy. The energies absorbed in the endothermic steps are identical in magnitude to the energies released in the exothermic steps for the same transition. ΔH Fusion = -ΔH Freezing
PDF Lecture Notes 2: Physical Equilibria - Phase Diagrams This diagram shows the regions of stability of different phases as a function of temperature and pressure. The interfaces between these regions will be the phase transition lines. The phase diagram for CO 2is shown below. Things to notice on the diagram. 1. Given a pressure and a temperature you can find the stable phase (gas, solid, or liquid) 2.




/phase-changes-56a12ddd3df78cf772682e07.png)


















Comments
Post a Comment