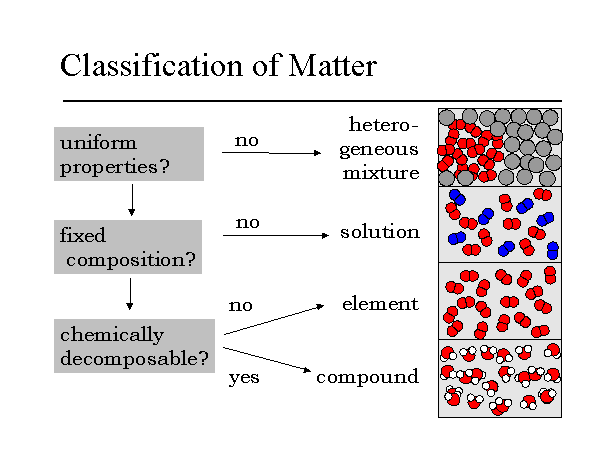
40 homogeneous mixture particle diagram
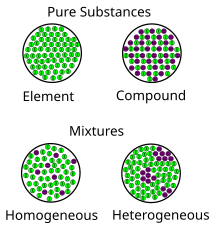
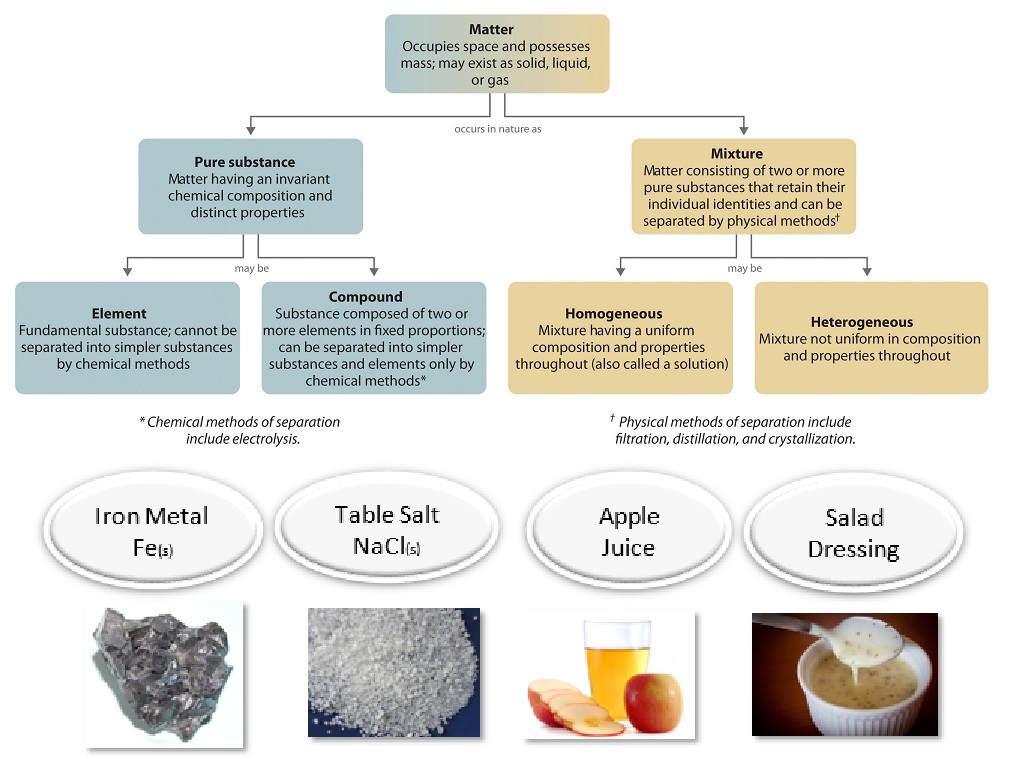
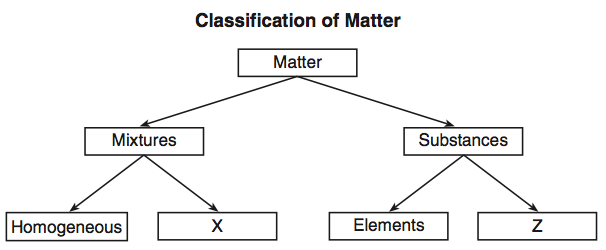
A mixture is a material made up of two or more different substances which are mixed but are not combined chemically. A mixture refers to the physical combination of two or more substances in which the identities are retained. Particle diagrams can also show different KINDS of mixtures: homogeneous & heterogeneous. Box A has a a. Homogeneous mixture of an element and a compound b. Heterogeneous mixture of an element and a compound c. Mixture of three elements d. Mixture of a liquid compound and a solid element 14. Which phrase best describes what is inside the box? a. Homogeneous mixture of two elements b. Homogeneous mixture of two compounds c. Heterogeneous mixture ...
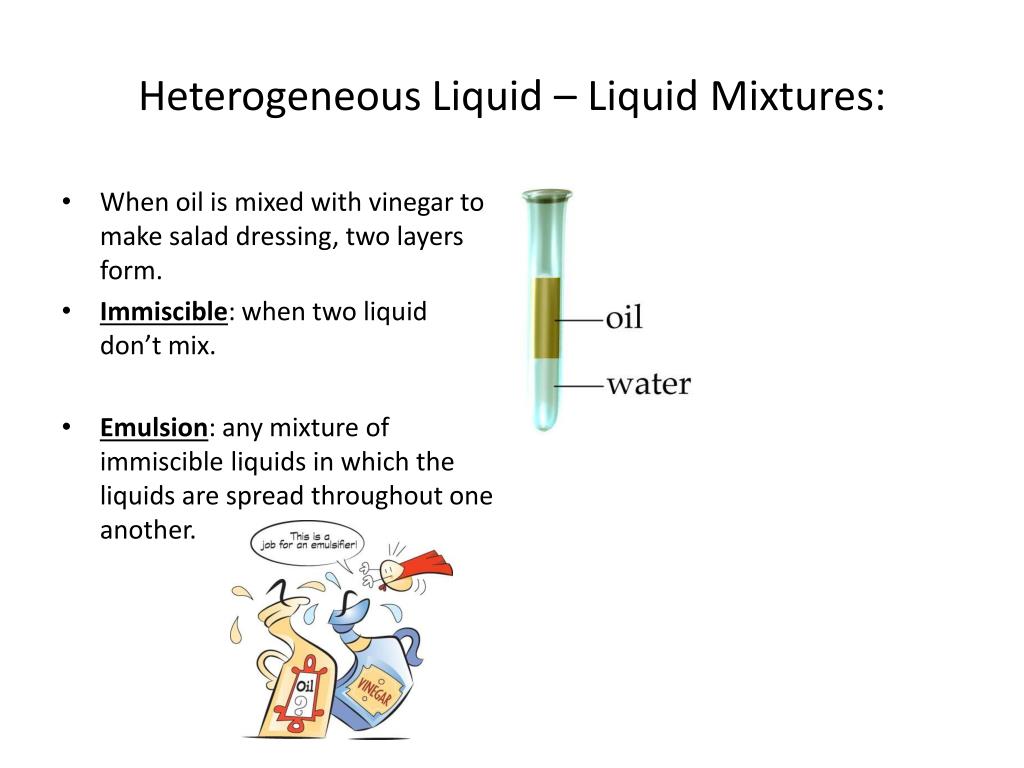
A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present. A mixture is an example of water. Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials. Stay tuned with BYJU'S to learn more interesting topics in Chemistry.

Homogeneous mixture particle diagram
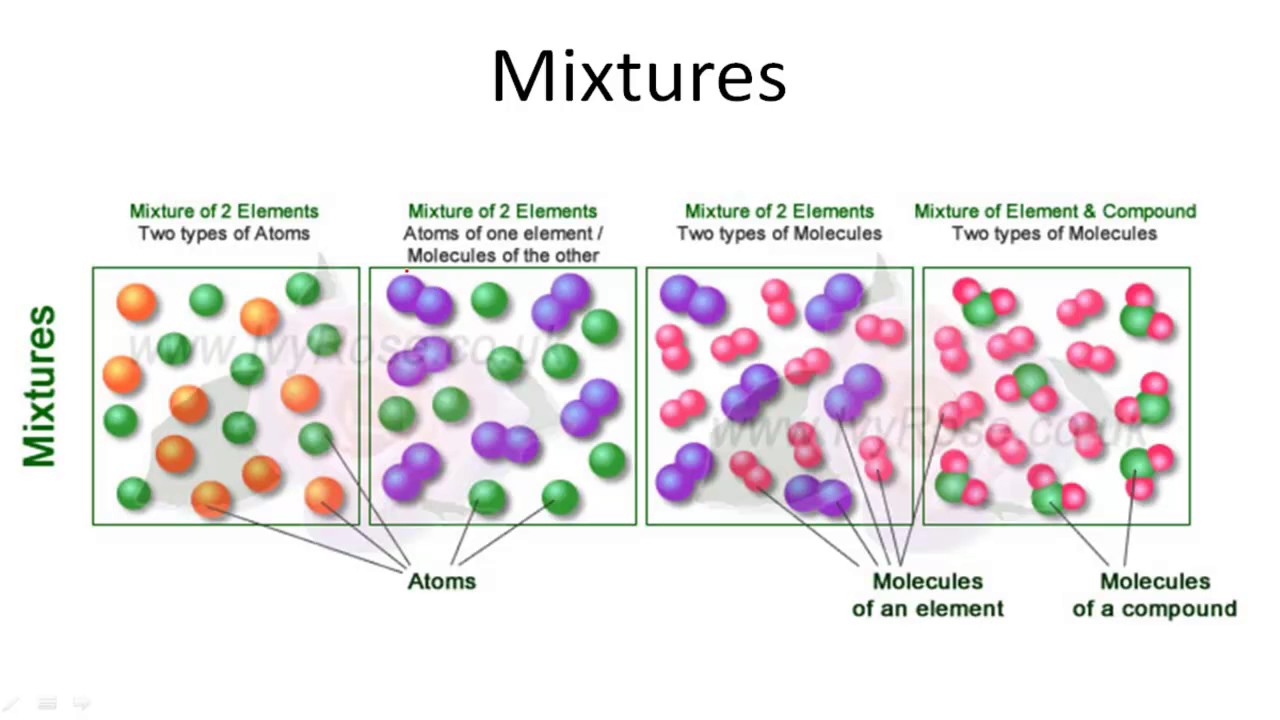
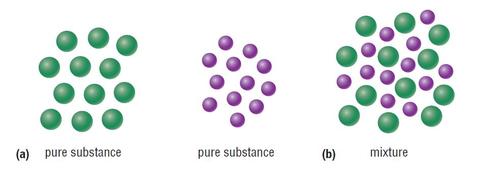
C= Mixture of elements Each circle represents an atom and each different color represents a different kind of atom. If two atoms are touching then they are bonded together. Then give an example for each (ex. #1 H 2 O) Objective: Differentiate between different types of particle diagrams Construct particle diagrams for pure substances and mixtures Heterogeneous mixture is a mixture with a non-uniform composition. When you mix two components that remain separate from each other, that mixture is called a Heterogeneous mixture. Concrete is an example of a Heterogenous mixture. A mixture of Cement and Water. A mixture of cold drinks and ice cube is also an example of a Heterogeneous mixture. (2) a mixture of elements (3) a mixture of compounds (4) a single compound 4 The particle diagram below represents a sample of matter. Which best describes the composition of the sample? (1) a heterogeneous mixture of elements and compounds (2) a heterogeneous mixture of elements (3) a homogeneous mixture of elements (4) a homogeneous mixture ...
Homogeneous mixture particle diagram. Mixtures can be homogeneous or heterogeneous. Solutions are always homogeneous. Heterogeneous mixtures are things like soil, fruit salad, where the composition is NOT uniform throughout the mixture. 3. The structure and arrangement of particles and their interactions ... Draw a particle diagram showing the change from solid wax to liquid wax ... DESCRIPTION PARTICLE DIAGRAM 1. ELEMENT 2. COMPOUND 3. MIXTURE OF ELEMENTS 4. MIXTURE OF COMPOUNDS 5. MIXTURE OF ELEMENTS AND COMPOUNDS Match the particle diagrams above with the correct description. Remembereach shape represents a different element Homogeneous and Heterogeneous Mixtures [classic] Use Creately's easy online diagram editor to edit this diagram, collaborate with others and export results to multiple image formats. You can edit this template and create your own diagram. Creately diagrams can be exported and added to Word, PPT (powerpoint), Excel, Visio or any other document. 48 Given the particle diagram: Which type of matter is represented by the particle diagram? (1) an element (2) a compound (3) a homogeneous mixture (4) a heterogeneous mixture. 2 : compounds 2 or more different elements chemically combined : 49 Which substance is an electrolyte? (1) O 2 (2) Xe (3) C 3 H 8 (4) KNO 3: 4 : look for an acid, base ...
represented using particle diagrams. •A particle diagram is a box in which coloured balls are draw to represent atoms or molecules. •These diagrams can represent elements and compounds, as well as their molecular composition by the types of balls and how they are connected. Element Compound Mixture of an Element and Compound Mixtures A mixture is made from different substances that are not chemically joined . For example, powdered iron and powdered sulfur mixed together makes a mixture of iron and sulfur. Classify each as a homogeneous mixture or heterogeneous mixture, or an element or compound (pure substance). a. water b. gold ... Label the arrows on the diagram below with the correct phase change processes. B) Draw a particle diagram representing each phase. Solid Liquid Gas . Title: CHEMISTRY I Author: George Flatau ... HOMOGENEOUS MIXTURE: • Substances are uniformly mixed • All solutions are homogeneous mixtures Example: Salt water NaCl (aq) ... substances and mixtures PARTICLE DIAGRAMS • Show how the forms of matter look in a simple diagram form TYPES OF PARTICLE DIAGRAMS ELEMENTS- MONATOMIC
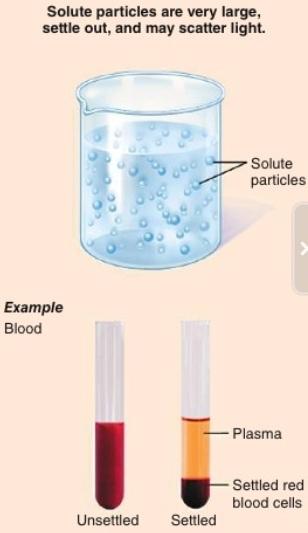
The diagrams above use arrows to represent the speed of a gas particle. Which of the diagrams best represents the speed of the particles of a gas at a fixed temperature, and why? Diagram 2, because the particles have a variety of different speeds. ... When methanol and water are mixed together, they form a homogeneous mixture. Based on the ... Particle size distinguishes homogeneous solutions from other heterogeneous mixtures. Solutions have particles which are the size of atoms or molecules - too small to be seen. A colloid is a homogeneous solution with intermediate particle size between a solution and a suspension. 1.Which two particle diagrams represent mixtures of diatomic elements? A) mass B)density C) length D) volume 2.At STP, which physical property of aluminum always ... The mixture is homogeneous and can be separated by filtration. B)The mixture is homogeneous and cannot be separated by filtration. The mixture is homogeneous and cannot be separated by filtration. Which particle diagram represents a mixture of an element and a compound? https://goo.gl/asBj4g
Chemistry Q&A Library 29) Which particle diagram above bestrepresents a mixture of compounds? A) A D) D C) C 30) The particle diagram below represents a sample of matter. Which best describes the composition of the sample? A) a mixture of elements a mixture of compounds D) a single element B) a single compound 31) Mixtures are defined as A) combinations of compounds and/or elements B) always ...
D) It is homogeneous, and its componentg are in different phases. 3. Distillation is a process used to separate a mixture of liquids based on different ELEMEÑT% A) boiling points C) freezing points B) densities D) solubilitieg 4. Which particle diagram represents a sample of oxygen gas at STP? one atom of oxygen A) B) D) 5.
2. The diagram above represents a mixture of NO 2(g) and N 2O 4(g) in a 1.0 L container at a given temperature. The two gases are in equilibrium according to the equation 2 NO 2(g) N 2O 4(g) Which of the following must be true about the value of the equilibrium constant for the reaction at this temperature? (A) K = 0 (B) 0 < K < 1 (C) K = 1
15. Draw particle diagrams of at least 6 particles in each diagram, to represent both ideas. Use these symbols to construct the particles in your diagrams: Iodine = Copper = Lisa's idea Bart's idea (the compound CuI) (mixture of Cu and I 2)
A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. The composition of the mixture is the same throughout. There is only one phase of matter observed in a homogeneous mixture at a time. So, you wouldn't observe both a liquid and a gas or a liquid and a solid in a homogeneous mixture.
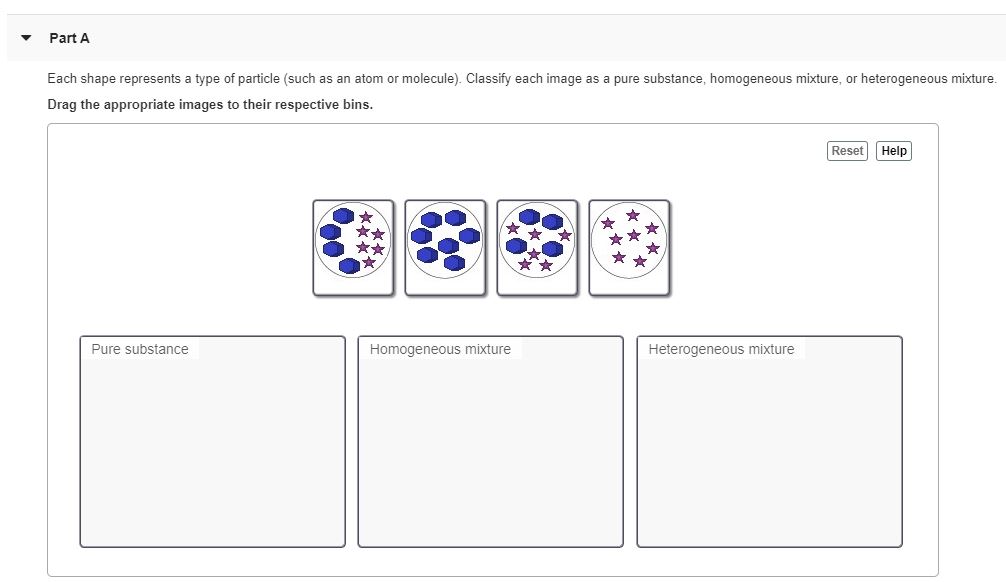
2013, High School Chemistry. Standard: 1.2 - Explain the difference between pure substances (elements and compounds) and mixtures. Differentiate between heterogeneous and homogeneous mixtures. Particle models of four different types of matter are shown in the diagram below. Identify which of the four models best represents a pure compound.
and Particle Diagrams Aim: To represent the different forms of matter using ... SUBSTANCES ELEMENTS and COMPOUNDS HOMOGENEOUS and HETEROGENEOUS MIXTURES Composition is variable. Composition is in fixed proportions. Diatomic Element-Found only combined in nature with itself. ... Mixture of 2 binary compounds Particle Diagrams One type of binary ...
16.) Draw a particle diagram for each of the following below. Then give an example for each. pure diatomic pure diatomic mixture of mixture of mixture of two element compound two elements an element & diatomic elements a compound & a compound 1.
• In homogeneous mixtures, the substances are completely mixed. This means that you cannot see the individual components. The mixture appears to be only one substance. • In heterogeneous mixtures, the substances are not completely mixed. ... Mixture particle diagram . Identify these diagrams: Identify this diagram:
17. Mixture 18. Heterogeneous Mixture 19. Homogeneous Mixture 20. Pure Substance 21. Particle Diagram 22. Chromatography 23. Filtration 24. Distillation 25. Scientific Notation Unit Objectives: When you complete this unit you will be able to do the following… 1) Classify types of matter 2) Draw particle diagrams to represent different types ...
We can draw particle diagrams of these three compounds as follows: H2O CO2 NaCl Compounds are made up of molecules (a specific ratio of chemically combined atoms). A mixture is a blend (physical change) of two or more substances. Homogeneous Mixtures are uniform throughout
(2) a mixture of elements (3) a mixture of compounds (4) a single compound 4 The particle diagram below represents a sample of matter. Which best describes the composition of the sample? (1) a heterogeneous mixture of elements and compounds (2) a heterogeneous mixture of elements (3) a homogeneous mixture of elements (4) a homogeneous mixture ...
Heterogeneous mixture is a mixture with a non-uniform composition. When you mix two components that remain separate from each other, that mixture is called a Heterogeneous mixture. Concrete is an example of a Heterogenous mixture. A mixture of Cement and Water. A mixture of cold drinks and ice cube is also an example of a Heterogeneous mixture.
C= Mixture of elements Each circle represents an atom and each different color represents a different kind of atom. If two atoms are touching then they are bonded together. Then give an example for each (ex. #1 H 2 O) Objective: Differentiate between different types of particle diagrams Construct particle diagrams for pure substances and mixtures






















Comments
Post a Comment